EU Food Information to Consumers (FIC) law changes: Impact on food supplement players
Let’s start with the presentation of ‘mandatory particulars’ – on-label statements the FIC demands by law.
FIC introduces minimum font size (1.2 mm) which will impact space requirements on food supplement labels that must include, as minimum, the warnings: ‘Do not exceed recommended daily dose’ or ‘Keep out of reach of young children’.
Food supplements containing allergens will need to follow the new allergens rules. In practice the most common solution for this type of product is to bold the name (or the part of the name) of the allergen in the list of ingredients.
Aspartame
When using additives, including those of the supplement’s capsule or casing, the name of the food additive category should always be presented as first. The decision whether to use the name or E number is quite important when a product contains aspartame. If this sweetener is designated in the list of ingredients only by reference to the E number, the additional labelling particular is: ‘Contains aspartame (a source of phenylalanine)’. Otherwise, if the designation is by the specific name, the additional labelling particular is: ‘Contains a source of phenylalanine’.
Caffeine
Food supplements fortified with caffeine placed on the market after December 12 this year should bear in the same field of vision as the product name the warning: ‘Contains caffeine. Not recommended for children or pregnant women’. The caffeine content should be expressed per portion as recommended for daily consumption on the labelling.
Mandatory particulars, such as the instructions for use, must be indicated with words and numbers, pictograms or symbols can only be an additional means. Words must come first.
Food supplements in powder or in other forms that require mixing of the product with water or other liquids should therefore include written instructions and not only depictions.
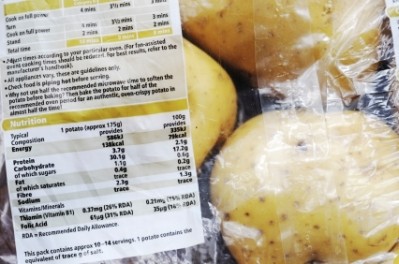
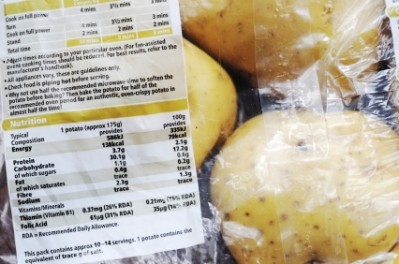
Nutrition declaration - ‘Reference Intakes’ or ‘Recommended Daily Allowances’?
Food supplements are exempted from the mandatory nutrition labelling that accompanies regular foods and drinks.
Nevertheless, if provided voluntarily, such information must comply with FIC. This applies to all seven mandatory nutrients: Energy, fat, saturates carbohydrate, sugars, protein, and salt.
Pursuant to 2002 EU Food Supplements Directive (FSD), the amount of nutrients or substances with a nutritional or physiological effect present in the product, have to be declared on the labelling.
For consistency, the Commission advises using the same terminology for food supplements as for other foods. Thus, ‘Reference Intakes’ should replace ‘Recommended Daily Allowance’.
Further preparations
As a general rule the nutrition declaration is required for the food as sold, but where appropriate, it can also relate to the food as prepared. Most food supplements bearing nutrition and health claims which should refer to the food as ready for human consumption.
Therefore when it comes to food supplements that bear nutrition and health claims (under the EU nutrition and health claims regulation (NHCR)) and need preparing before consumption, nutrition labelling relating to the food as prepared might be more convenient for both consumer and producer.
On the other hand where there are various preparation options – such as with water, with milk or with juice, such obligation may force a choice of only one option.
For nutrition declaration purposes as well as the declaration of the vitamins and minerals present in food supplements, the names listed in Annex XIII of FIC should be used. For instance, ‘thiamine’ cannot be replaced with B1 or ‘folic acid’ with B9.
COOL - ‘Produced in the USA?’
The indication of the country of labelling origin (COOL) or of the place of provenance is mandatory for certain foodstuffs (e.g. beef, honey, olive oil).
It also should be provided whenever its absence is likely to mislead consumers as to the true country of origin or place of provenance of that product. In some cases, producers indicate the origin of a food on a voluntary basis to draw consumers’ attention to the qualities of their product (e.g. ‘Product of USA’, ‘Produced in EU’).
Such indications should however comply with EU harmonised criteria. Where the country of origin or the place of provenance of a food is given and where it is not the same as that of its primary ingredient (i.e. ingredient that represents more than 50 % or which is usually associated with the name of the food), COOL of the primary ingredient must be indicated as different to that of the food.
With regard to food supplements, the origin of plant extracts or even of the capsule may have impact on the correct labelling.
Izabela Tańska and Sebastián Romero Melchor are partners at the K&L Gates legal firm in Brussels, Belgium.