EHPM multitiered proposal offers solution to ‘on-hold’ botanical claims bottleneck
In a briefing paper released last month details of the approach, which relies on a grading format, demonstrates a possible way to rank botanical health claims, ‘from the certain to the plausible.’
The association adds the approach enables claims to be evaluated by referencing specified levels of evidence and to use qualified language to communicate the evaluation outcomes in a ‘useful, honest, accurate and meaningful way.’
“This approach logically involves dealing with uncertainty, but the methodology proposed shows that this can be achieved without compromising the required stringency,” the paper adds.
The EPHM’s efforts attempt to navigate a coherent path in the never-ending saga that has resulted in over 2000 health claim applications placed on-hold.
The pause in proceedings is due to the body responsible for assessing health claims, the European Food Safety Authority (EFSA) and its stance on the methodologies used in authorising claims.
EFSA have deemed current approaches inadequate for claims associated with more complex foodstuffs such as those obtained from plants and other vegetative organisms or ‘botanicals.’
At present, the industry is unable to provide information on the health benefits associated with such food products, leading to fears that consumers will turn to uncontrolled, unregulated sources such as the Internet for both information and supply.
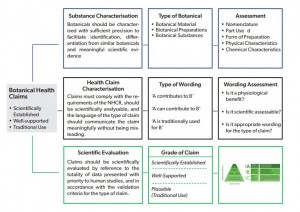
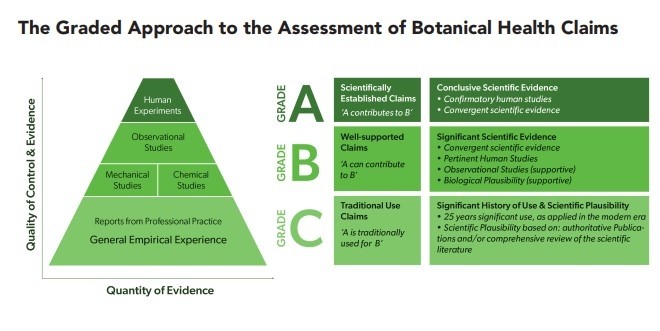
Grades A-C
At the centre of the EHPM’s model is its harmony towards the requirements set by the Nutrition and Health Claims Regulation (EC 1924/2006) (NHCR), which sets out rules for using health claims on foods.
The approach, which also considers the systematic procedures already adopted by EFSA, categorises scientifically established health claims, similar to those already authorised by the food authority, as ‘Grade A.’
“By qualifying the quality and scale of ‘convincing’ clinical studies to reflect what is practically achievable for complex botanicals, a modified approach is proposed for confirming a cause effect relationship between a botanical foodstuff and health,” the paper states.
Claims validated in this way are expressed with a high degree of certainty; e.g. ‘Bacopa monnieri improves cognitive functions and memory’.
Conditions of use of the claim should be defined e.g.: Minimum 120 mg Bacosides per day brought by Bacopa monnieri extract standardised in Bacosides (USP method).”
A ‘Grade B’ health claim refers to scientifically well supported health claims that are based on significant developments in modern science and experience.
“To be considered scientifically well-supported, botanical health claims must be based on a convergent body of evidence that includes detailed chemical profiling of the botanical, identification and study of active constituents in the laboratory.
“The evidence must also include observation of the effects of the botanical in human beings, and well-conducted clinical trials which are of sufficient quality and scale to demonstrate an effect, but not enough to be considered conclusive.”
The EHPM gave a Grade B health claim example as, ‘Isoflavones of red clover (trifolium pratense) can contribute to lowering LDL-cholesterol in postmenopausal women.
‘Condition of use of the claim should be defined e.g.: a dose of 50 to 80 mg/day of red clover’s isoflavones.’
Finally, a Grade C health claim would include traditional use health claims that have been used for at least one generation and which are considered to be scientifically plausible.
“Traditional use health claims are based on traditions of use substantiated by bibliographic evidence and/or industry data; the scientific plausibility of such claims must also be demonstrated by reference to recognised publications (e.g. Monographs), or by a critical appraisal of a comprehensive review of the scientific literature.
“For example, ‘Thyme (thymus vulgaris) is traditionally used to support the health of the respiratory system.”
Practical assessment
The association also offers a practical assessment of the graded health claims for botanicals (see right) adding that, “Any EU approach to health claims on foods must adhere to the requirements of the Nutrition and Health Claims regulation (NHCR),” they say.
“Consumers must be protected from being misled and a high-quality scientific assessment must be carried out.
“EHPM has therefore identified how the Graded Approach to health claims for botanical food stuffs, as particularly applied to food supplements, can be evaluated using the general approach already adopted by EFSA.”